April 21, 2020

There is a growing need to obtain comprehensive, longitudinal data to understand medical device safety and effectiveness in the post-market setting. However, investigators face several challenges, including difficulty engaging patients in research and collecting the necessary follow-up information.
The Yale University – Mayo Clinic Center of Excellence in Regulatory Science and Innovation (CERSI) was established approximately three years ago by Joseph Ross, MD, MHS, and Nilay Shah, PhD, through a grant awarded by the U.S. Food and Drug Administration (FDA). Now, in 2020, the Yale-Mayo Clinic collaboration helps lead a host of high-impact research projects using Real-World Data (RWD) sources to advance regulatory science, one of which is designated as a NESTcc Demonstration Project: Post-Market Medical Device Surveillance with a Novel mHealth Platform. This designation signals to the broader medical device community that the study contributes critical value to the field of Real-World Evidence (RWE) within the medical device ecosystem.
How Can a Mobile Health Platform Be Leveraged?
Mobile Health or mHealth is a term that refers to the use of mobile and wireless technologies to support the achievement of health objectives in the clinical setting as well as in research settings.
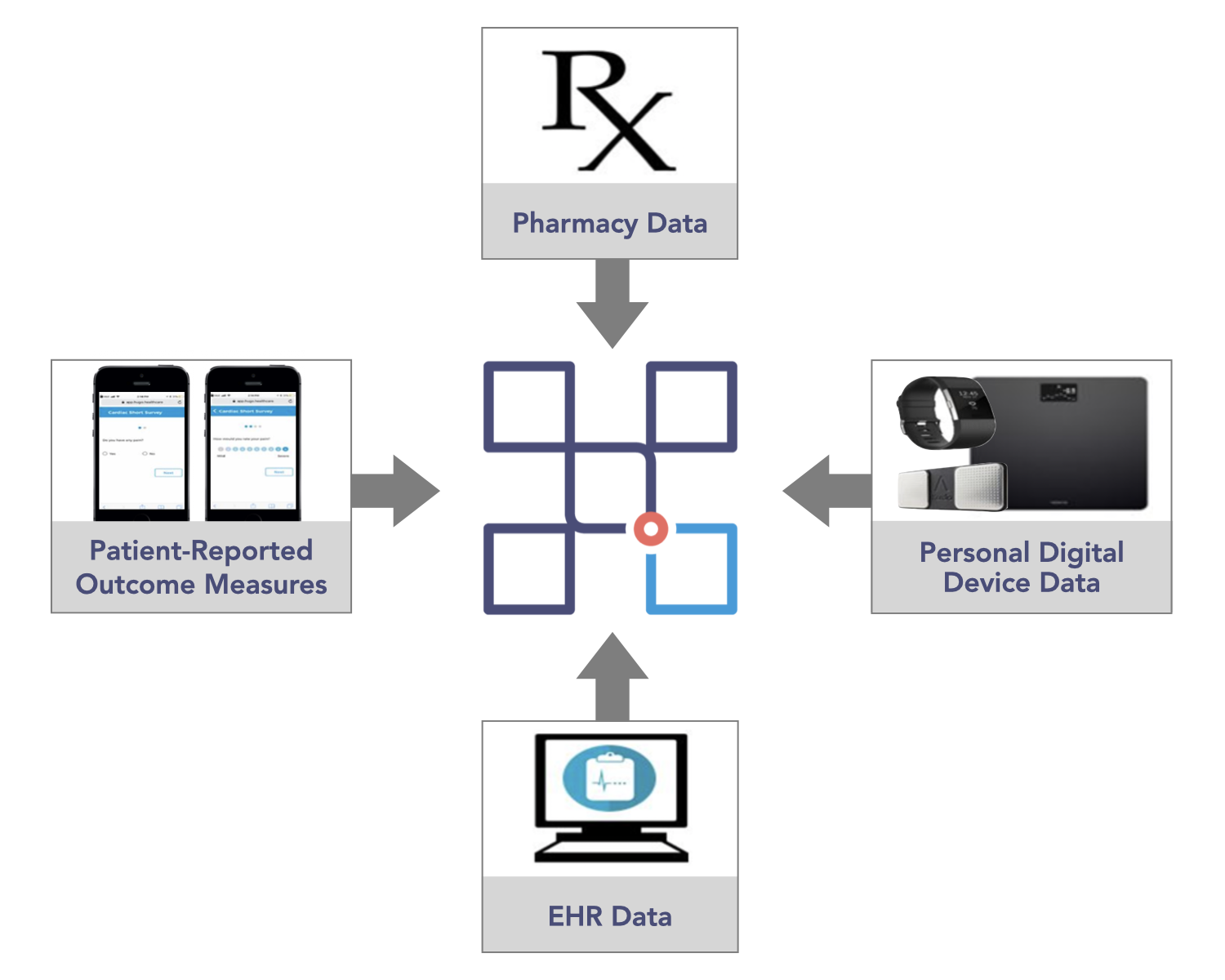
Given mHealth’s potential to transform the face of health service delivery, the CERSI project sought to test the feasibility of using a novel patient-centered health data-sharing platform, Hugo, to obtain and aggregate multiple real-world health data sources (i.e., electronic health records (EHR) from health systems, pharmacies, personal digital devices, and patient-reported outcome measures collected through surveys) in a prospective cohort of patients who received atrial fibrillation ablation or bariatric surgery – see Table 1.
A Hugo account was created for all patients once they were enrolled to enable access to the four sources of data that they shared with the research team over an eight-week follow-up period.
| Study Design | Observational; mHealth Pilot-Test Feasibility Study |
| Enrollment | 60 participants (30 each at Yale-New Haven Hospital and Mayo Clinic) |
| Target Population | Patients >18 years old who received a sleeve gastrectomy or gastric bypass and patients who received catheter-based atrial fibrillation ablation |
| Study Design | Prospective Cohort Study |
| Target Follow-Up Duration | 8 weeks |
Table 1. Summary of Study Design and Implementation Strategy
For more details regarding the project study design, please visit ClinicalTrials.gov (NCT03436082).
Study Illustrates Key Potential of mHealth for Postmarket Device Evaluation
The CERSI project found the innovative Hugo platform helps to obtain comprehensive, longitudinal data to understand the safety and effectiveness of a specific medical device in the post-market setting that other RWD data sources are unable to accomplish. Some of the most critical lessons demonstrated through their work showed that mHealth technologies can enable:
- Successful aggregation of multiple real-world data sources in near real-time
- Data collection and analysis are timelier, and data completeness is more likely.
- Patients to have more access to their data and connect data from disparate sources
- Research efforts can be enhanced by obtaining accurate, structured data directly from patients that are generated in their everyday lives
- Ten patients linked electronic health records from additional portals in addition to Yale-New Haven Hospital and Mayo Clinic; data were available from a total 13 electronic health records
- Twenty-four patients used CVS or Walgreens pharmacy, and connecting these pharmacy sources enabled them to share their medication data.
- High rates of weekly use of Fitbit, Withings scale, and Kardia Mobile contributed relevant post-procedure data, such as increasing activity over time and weight loss, etc.
- Increased patient engagement in research and agency over their healthcare data
- Gives patients access to their health information when they need it most and in a way they can best use it.
- More than 80% of patient-reported outcome measure questionnaires were completed, contributing to improved understanding of patient symptoms, recovery, and status
Why Should NESTcc Encourage mHealth Tools for RWE?
Although the Yale-Mayo CERSI program continues to be successful in using mHealth platforms to study device performance in post-market settings, data capture/access and data privacy challenges remain significant barriers to broader adoption of these patient-facing tools.
As a public-private entity dedicated to scaling up the use of RWE in device surveillance, NESTcc is in a unique position to communicate with medical device stakeholders and encourage access to and linkage of data sources, such as EHR, pharmacy data, registries, claims data, and others as well as stimulate UDI adoption for improved data accuracy, consistency, and completeness. Dr. Shah highlighted the Interoperability and Patient Access final rule recently issued under the MyHealthEData initiative, which requires CMS-regulated payers to give patients easier access to their claims and encounter information.
The concept of patient-centered care and patient-centered research is increasingly considered an essential component of high-quality health care systems. mHealth tools improve the paradigm so that patients engage as research partners instead of only data providers.
Buoyed by the success of this project, CERSI is delighted that NESTcc is supporting two Round 2 Test-Cases that use a similar approach:
- Use of RWD to Evaluate Clinical and Patient Outcomes by Use of a Prescription Digital Therapeutic (delivered via mobile app) for the Treatment of Insomnia and Depression)
- Effect of the Apple Watch ECG and Irregular Rhythm Notification Detection Features on Patient-Reported Outcomes and Clinical Utilization: A Randomized, Controlled Trial
The manuscript for this Demonstration Project was published in npj Digital Medicine on April 20, 2020.
“…health insurance and CMS would be the next frontier of data access – having the ability to pull data directly from the health system portals and link them to pulled claims data without compromising patient confidentiality would likely lead to better healthcare outcomes.”
Nilay Shah, PhD
Demonstration Project Snapshot
| Project Name | Aggregating Multiple Real-World Data Sources using a Patient-Centered Health Data Sharing Platform: An 8-week Cohort Study among Patients Undergoing Bariatric Surgery or Catheter Ablation of Atrial Fibrillation |
| Project Start/End Date | 2017-2019 |
| Study Type | Observational Prospective cohort |
| Total Product Life Cycle | Post-Market Surveillance |
| Disease Area | Cardiovascular and Bariatric Surgery |
| Data Sources Used | EHR, Pharmacy, Patient-Generated Data, Patient-Reported Outcome Measures (PROMs) |
| Organizations | Yale–Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) |
| Additional Collaborators | Food and Drug Administration (FDA) – Center for Devices and Radiological Health (CDRH), Johnson & Johnson, and Hugo Health |

Joseph Ross, MD, MHS
Principal Investigator

Sanket S. Dhruva, MD
Principal Investigator

Nilay Shah, PhD
Principal Investigator