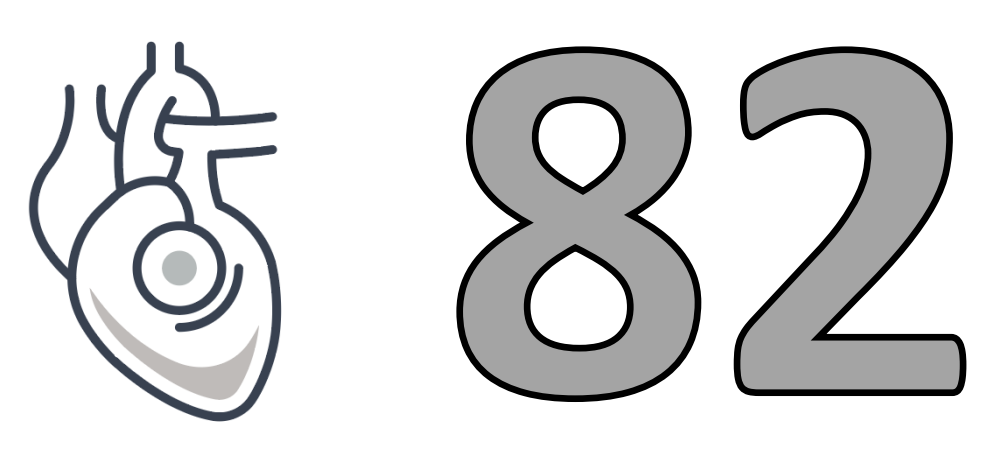Real-World Data (RWD)
Real-world data are the data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources. These include, but are not limited to, electronic health records (EHRs), claims, registries and patient-generated data.
Real-World Evidence (RWE)
Real-world evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of RWD.
Number of RWE Examples
In March 2021, the U.S. Food & Drug Administration (FDA) published a selected set of examples of medical device regulatory submissions using RWE. While not inclusive of all submissions or regulatory decisions that used RWE, the sample showcases the various uses of RWE as valid scientific evidence.
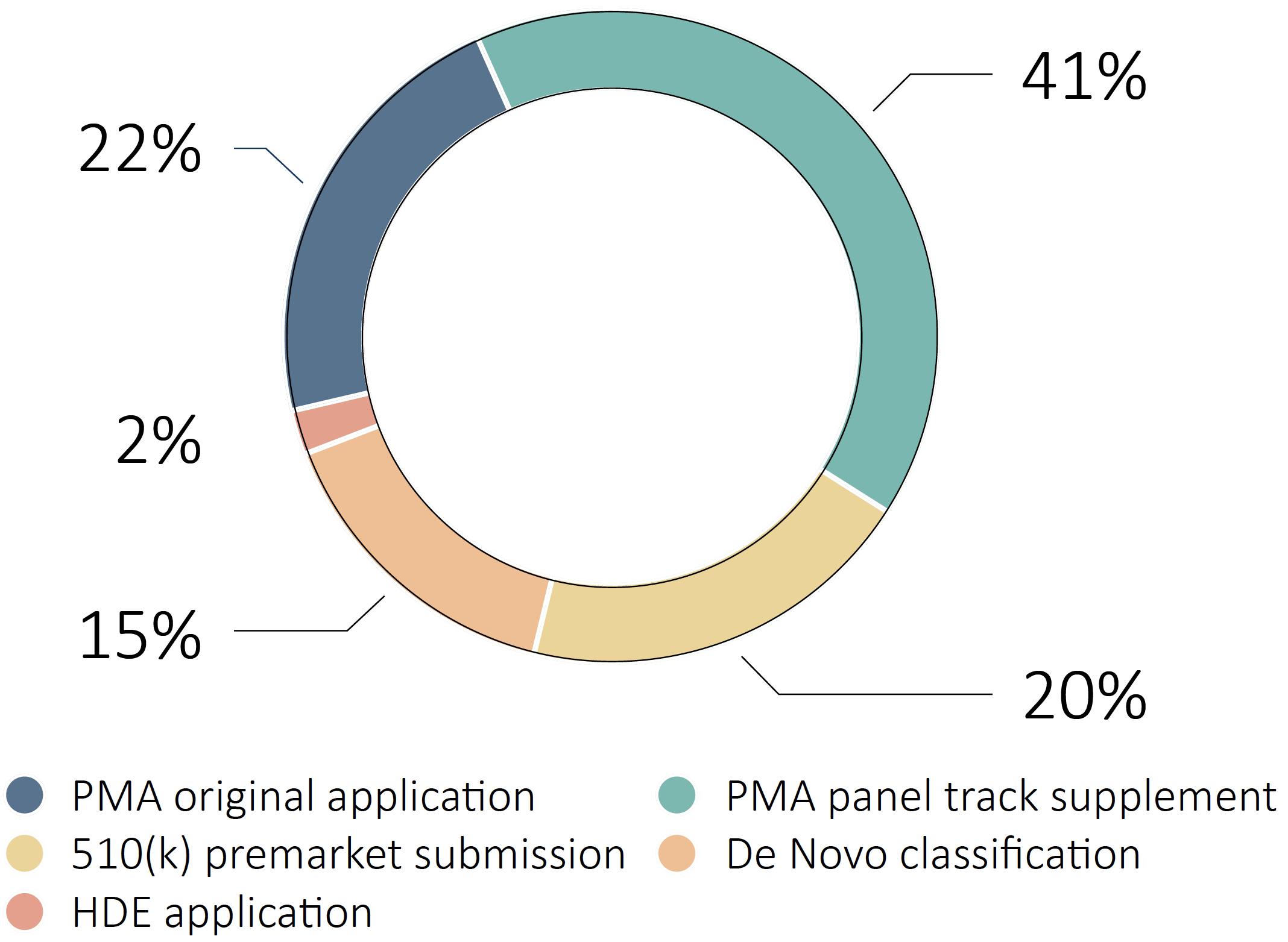
By Regulatory Pathway
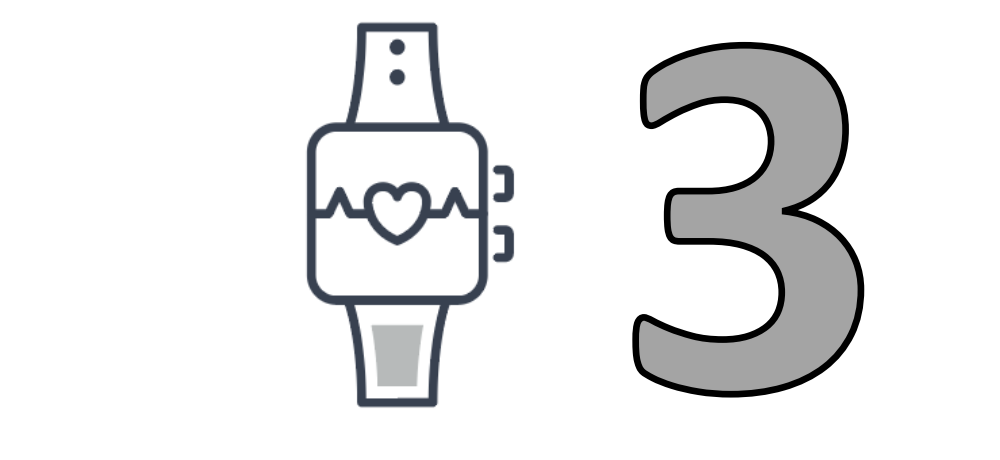
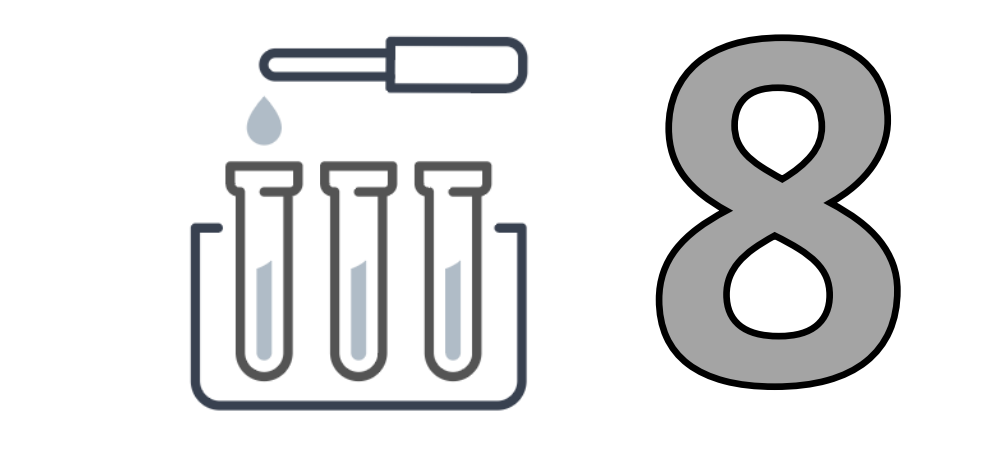
By Device Type
Total therapeutic devices
of the therapeutic devices were digital devices
In vitro diagnostics
By Area of Specialty
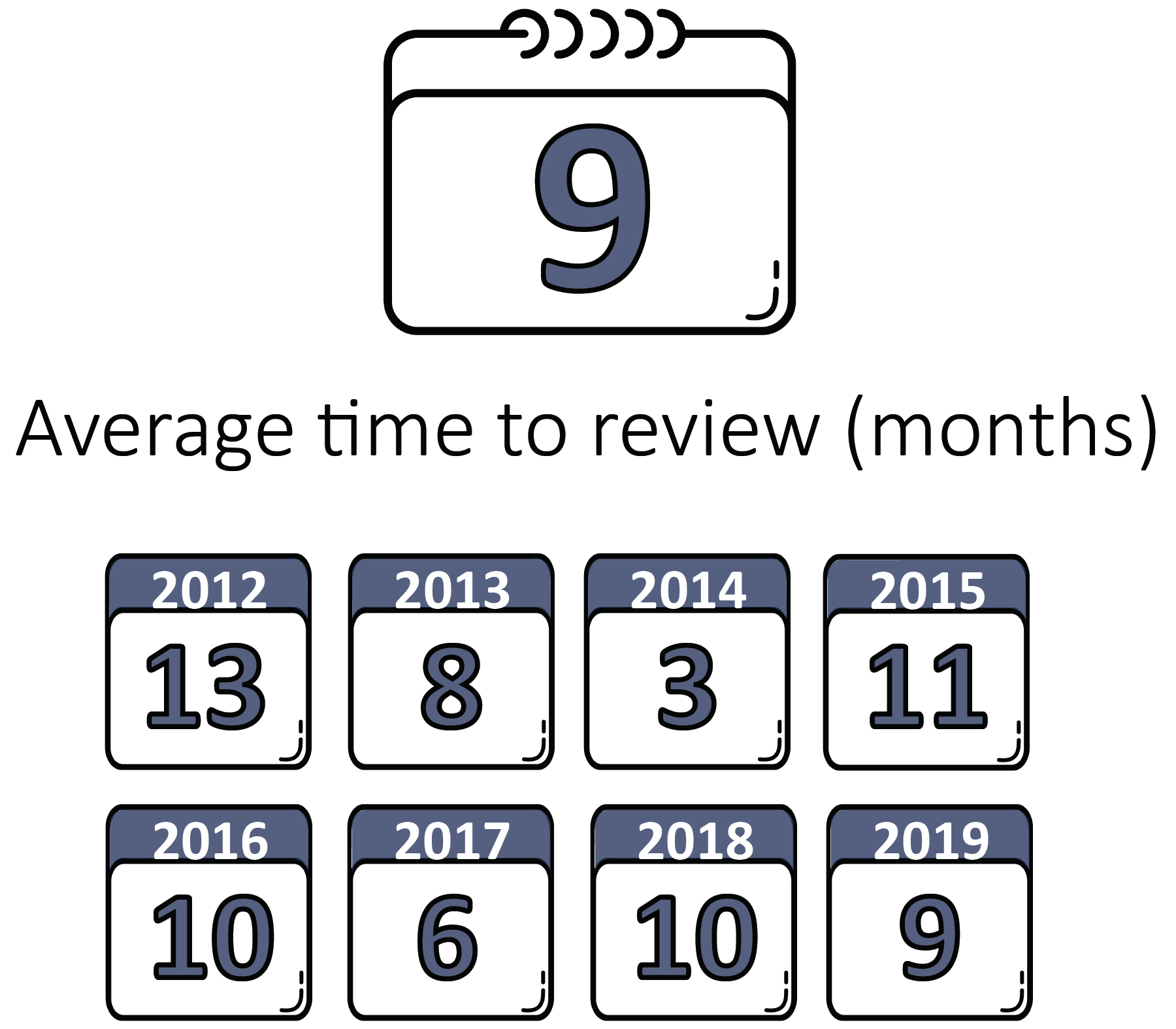
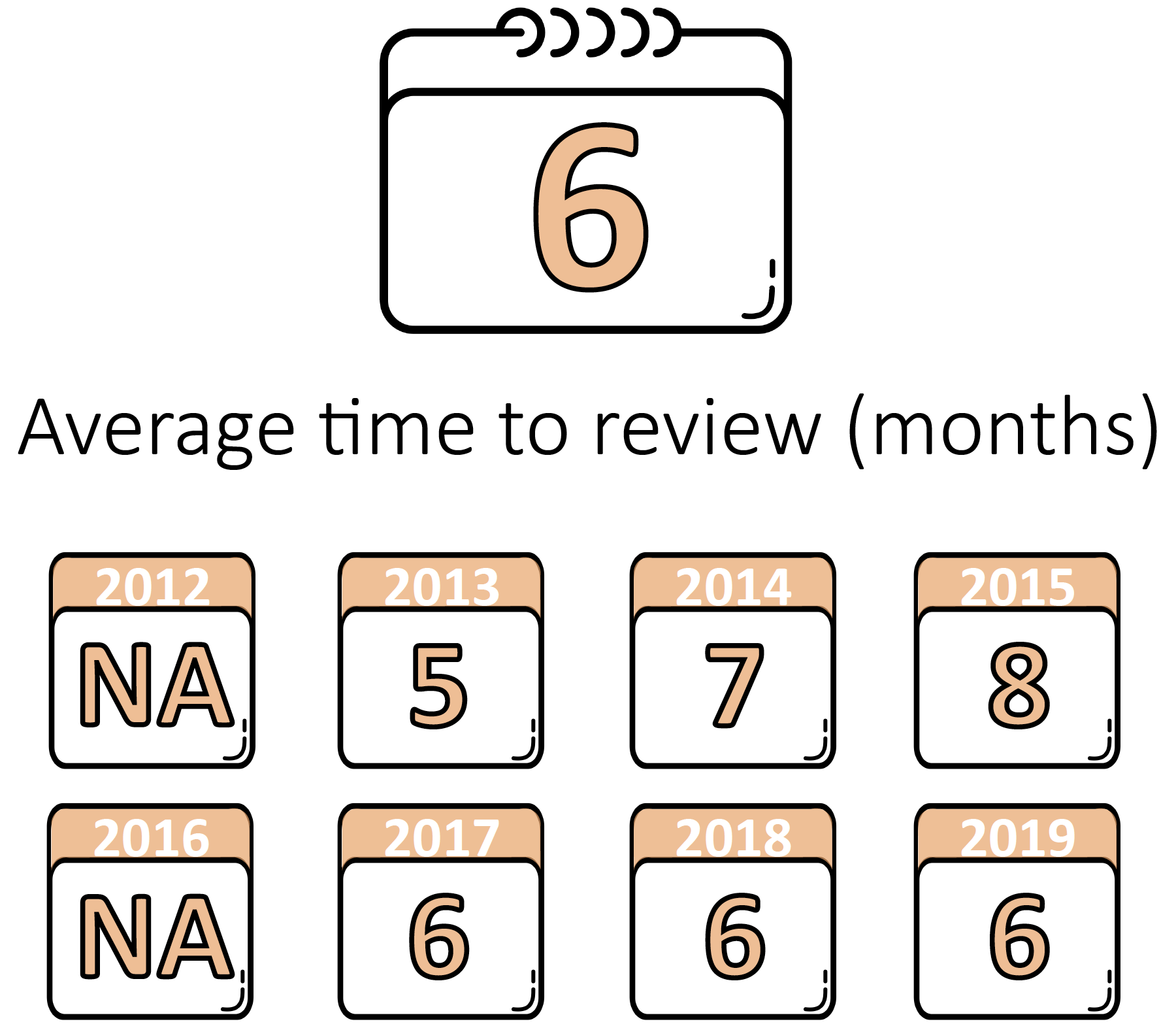
Average Time to Review
Among the 90 publicly available RWE examples from 2012 to 2019 published by FDA, the average time to review was nine months for PMA/HDE devices and six months for 510(k)/De Novo devices. The calendar years at right reflect the years in which devices received regulatory decisions.
58 PMA/HDE Examples
32 510(k)/De Novo Examples
Please note that review times are based upon the regulatory application receipt and decision dates publicly available in the FDA report and database. The use of RWE may not have been applied until a time period after device clearance or approval (e.g., as part of PAS requirements following a decision).
Interested in more real-world experience with RWE?
Contact us to learn about our 21 RWE Test-Cases and collaborative dialogue with Offices of Health Technology to support alignment on RWE evidentiary requirements.